Disposable Medical Radial Artery Compression Tourniquet Devices CE / ISO
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
T1 CE&ISO Disposable medical eco-friendly latex free tourniquet Surgical Arterial &n
T1 CE&ISO Disposable medical eco-friendly latex free tourniquet Surgical Arterial
Description:
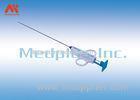
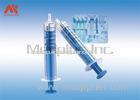
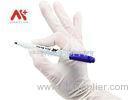
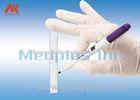
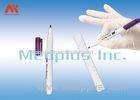
This is a compression device to assist hemostasis of the radial artery. It is used for the fistulation patients who are post hemodialysis operation and the ones who are post transradial inwasive monitor for blood pressure.It is intended for ICU,Anesthesiology Department, Hemodialysis Department and so on.
Why to Choose T1 Helix radial artery compression tourniquet?
1. T1the indicator design can be see the pressure intuitively
2. T1 the indicator is from 150mmhg to 250mmhg, there are two locations, the first is 150mmhg to 200mmHg, the second is 200mmHg to 250mmHg
3. We have spring design, this is our patent .When you use the tourniquet in patient and add the pressure, spring design avoiding shake.
4. Presure value indicates design is easy to know how much pressure was pressing to patient, avoiding pressing more or less pressure to patient.
5. The turn cap design is easy to add or reduce the pressure, not need syringe or other tool to help.
6. Soft cushion design. This cushion is very soft, reduce ache.
Characteristics:
1. Transparent design for precise operation
2. Stop bleeding by simply adjusting the band, or by adjusting the turn cap.
3. Colored marker helps medical staff distinguish the pressure thus makes bleeding point comfort.
4. The device can press on the puncture point precisely to stop bleeding.
5. Cushion made up of special material; no need to fix the patient’s arm; greatly reduce the patient’s pain.
Ethylene oxide sterilization without toxicity.
6. Convenient and effective, obtained CE Certificate and FDA Medical Devices Listing.
Specification: T1 type
Intended Use
This is a compression device to assist hemostasis of the radial artery after a transradial procedure.
Precautions & warnings
* This device is suitable for patients whose wrist circumference is 140-240mm
* The medical staff should keep the patients wrist straight and the patient should avoid strenuous activity of the arm when this device is used .After the procedure the patient should refrain from bending the wrist for 2 hours or move the wrist for 4-6hours.
* Depending on the patient’s condition and the degree of cushion pressure, an adverse event including artery occlusion,hypodermic haematoma, haemorrhage, pain, or numbness may occur, check the progress of the haemostasis and adjust the pressure accordingly by turning the turn cap.
* Patient should not be left unattended while this device is in use
* If there is itching or redness of the skin while compressiong, stop using and treat appropriately.
* This device should be used immediately after opening the package .Don’t turn the turn cap excessively in order to avoid stick fast or screw loose.
* Do not use this device if unit package or the device has been damage or soiled .Disposed of safely and properly as medical rubbish after used.
* Do not soak or wipe with agents containing organic solvents. Such agents may cause damage to this device.
* Ethylene Oxide sterilized, for single use only, do not re-sterilized or reuse. Re-sterilize or reuse may bring infection.
* Avoid exposure to water, direct sunlight, extreme temperature, or high humidity during storage.
Sterilization validity: 3 years.
Mode and packing:
Model | Description | packing | Carton size |
Tourniquet( Radial Artery Compression Device) | |||
010211 | T1 Type, used for assisting hemostasis of the radial artery after a trans radial procedure | Single packing , EO sterilized 10pcs/bx,100pcs/CTN | 63*38*23cm |
010212 | T2 Type, used for assisting hemostasis of the radial artery after a trans radial procedure | Single packing , EO sterilized 10pcs/bx,100pcs/CTN | 63*38*23cm |
010221 | Hemodialysis Type, used for hemodialysis operations | Single packing , EO sterilized 10pcs/bx,100pcs/CTN | 63*38*23cm |
0102A21 | Soft and transparent pad |
| 63*38*23cm |
About us:
There are 10W cleaning room quality is guarantee and accepted by our customers.
Cost effective control, Department of technology and QC and sales team
80% products exportation, Main customers from EU, US, AU, AF, AS, etc
100 % Factory price, Quantity discount, Flexible terms of payment, fast delivery in safe, timely after sale service.
Related Search
Find more related products in following catalogs on Hisupplier.com

Company Info
Medplus Inc Company [China (Mainland)]
Business Type:Manufacturer
City: Guangzhou
Province/State: Guangdong
Country/Region: China (Mainland)
You May Like:
Sitemap XML
About HiSupplier Help Center Customer Service Friend Links Site Map Archives
Browse by: China Suppliers - Hot Products - Products Directory - Offers Directory - Suppliers Directory - Buyers Directory
Language Option: العربية - Nederlands- Français- Deutsch- Italiano- 日本語- 한국의- Português- Pусский- Español